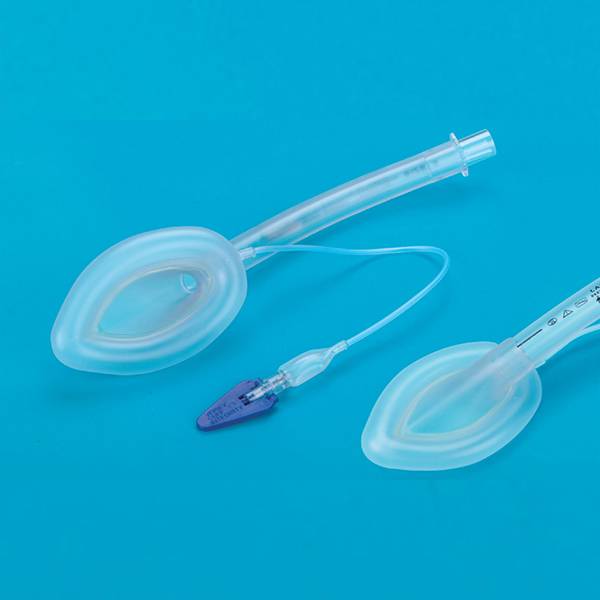
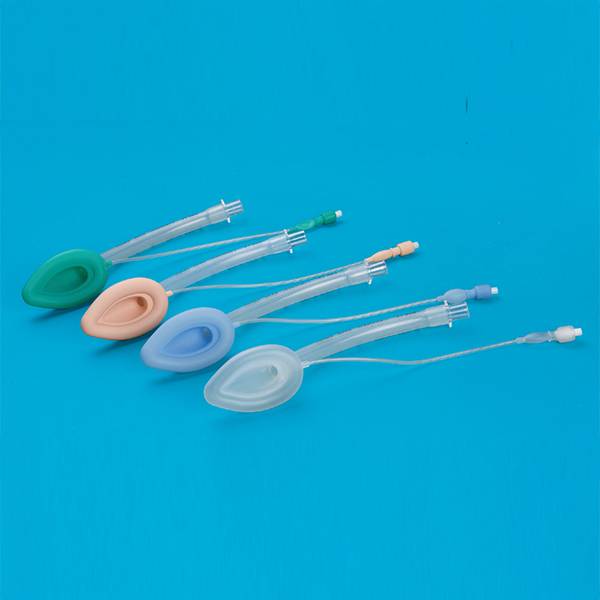
Guedel Airway
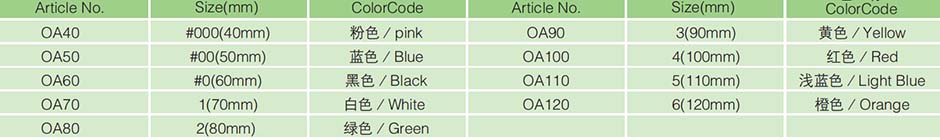
Packing:50 pcs/box, 10 boxes/carton
Carton size:48 × 32 × 55 cm
This product is suitable for clinical patients with airway obstruction, maintain airway patency.
|
Model specifications(cm) |
3 |
3.5 |
4 |
4.5 |
5 |
5.5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Nominal specification (nominal length)(cm) |
3 |
3.5 |
4 |
4.5 |
5 |
5.5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
The product is composed of a tube body, inner tube of bite plug (no bite). The tube body and the polyethylene material used by bite plug tube medical grade (PE), polypropylene (PP) material. Product sterility, If the use of ethylene oxide sterilization, ethylene oxide residue in the factory should be less than 10μg/g.
1. In the insert oropharyngeal airway before reaching the depth of anesthesia satisfaction, in order to suppress the throat reflex.
2. Select the appropriate oropharyngeal airway.
3.Open the patient's mouth, and placed in on the root of the tongue, tongue upward, left posterior pharyngeal wall and the oropharyngeal airway into the mouth, until the end of 1 prominent incisors 1- 2cm, the front end of the oropharyngeal airway will reach the oropharyngeal wall.
4. Both hands hold the jaw, tongue left posterior pharyngeal wall, Then the flange of the two side of the thumb is placed in the hands of the edge of the oropharyngeal airway, push down at least 2cm, Flange until the oropharyngeal airway reaches above the lip.
5. Relax the condyle of the mandible, and make it back to the temporomandibular joint. The oral examination, in order to prevent the tongue or lip is clamped between the teeth and oropharyngeal airway.
Patients with lower respiratory tract obstruction.
[Untoward effect] nothing.
1. Before use, please select the correct size according to the age and weight, and check the quality of the product.
2. Please check before use, such as found in single (packaging) products have the following conditions, is prohibited to use.
a) The effective period of sterilization failure;
b) The product is damaged or a single piece of foreign matter.
3. This product for clinical use, operation and use by the medical staff, after the destruction.
4. In the use of the process, should be timely monitoring of the use of the situation, if there is an accident, should immediately stop using.
5. This product is sterile, sterilized by ethylene oxide.
[Storage]
Products should be stored in relative humidity of not more than 80%, no corrosive gas and good ventilation clean room.
[Date of manufacture] See inner packing label
[Expiry date] See inner packing label
[Registered person]
Manufacturer: HAIYAN KANGYUAN MEDICAL INSTRUMENT CO.,LTD.

 中文
中文