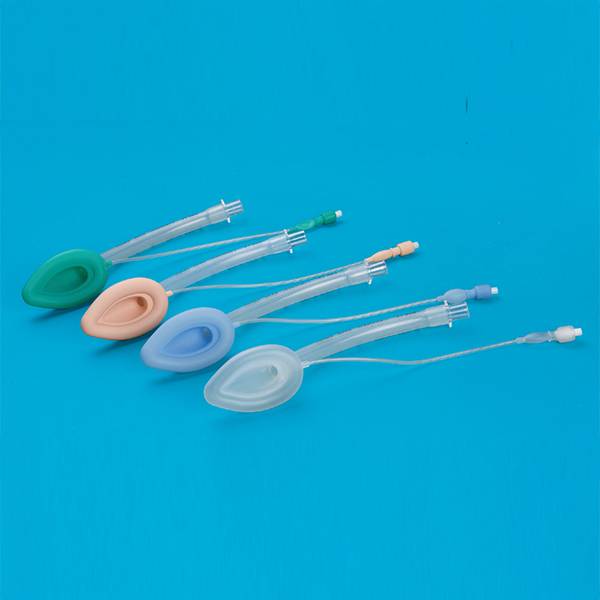
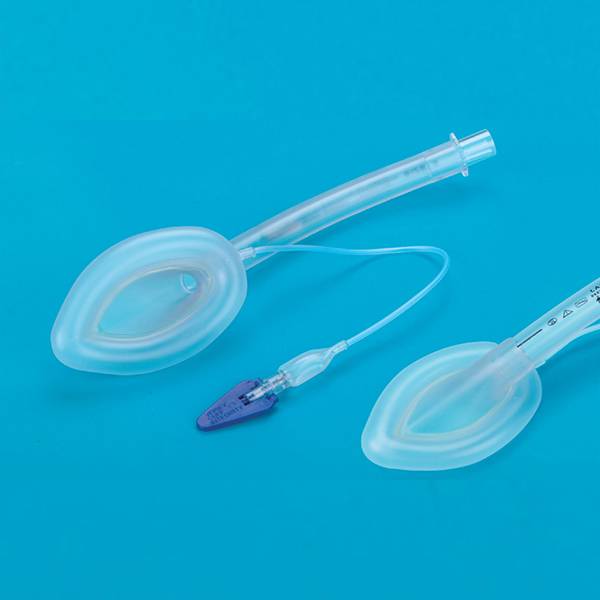
Laryngeal Mask Airway for Single Use
Packing: 5 pcs/box. 50 pcs/carton
Carton size: 60x40x28 cm
The product is suitable for use in patients who need general anesthesia and emergency resuscitation, or to establish short-term non deterministic artificial airway for patients who need breathing.
This product according to the structure can be divided into ordinary type, double strengthened type, ordinary type, double reinforced four types. The ordinary type ventilation tube, cover bag fittings, inflatable tube, indicating airbag, joint and inflatable valve; reinforced by ventilation tube, cover bag connector, an aeration pipe. Indication of the air guide rod, (can not), and joint charge valve; double ordinary type by the ventilation tube, drainage tube, cover bag fittings, inflatable tube, indicating airbag, joint and inflatable valve; double pipe reinforced by ventilation pipe, drainage pipe, cover bag fittings, inflatable tube, indicator the airbag, the connecting sleeve pad, guide rod (no), joint and a charge valve. Strengthen and double reinforced laryngeal mask on the inner wall of the trachea with stainless steel wire products. To strengthen the ventilation tube, drainage tube, cover bag connecting piece, the connecting sleeve pad, inflatable tube, air bag adopts instructions made of silicon rubber material. If the product is sterile; ring Oxygen ethane sterilization, ethylene oxide residues should be less than 10μg/g.
|
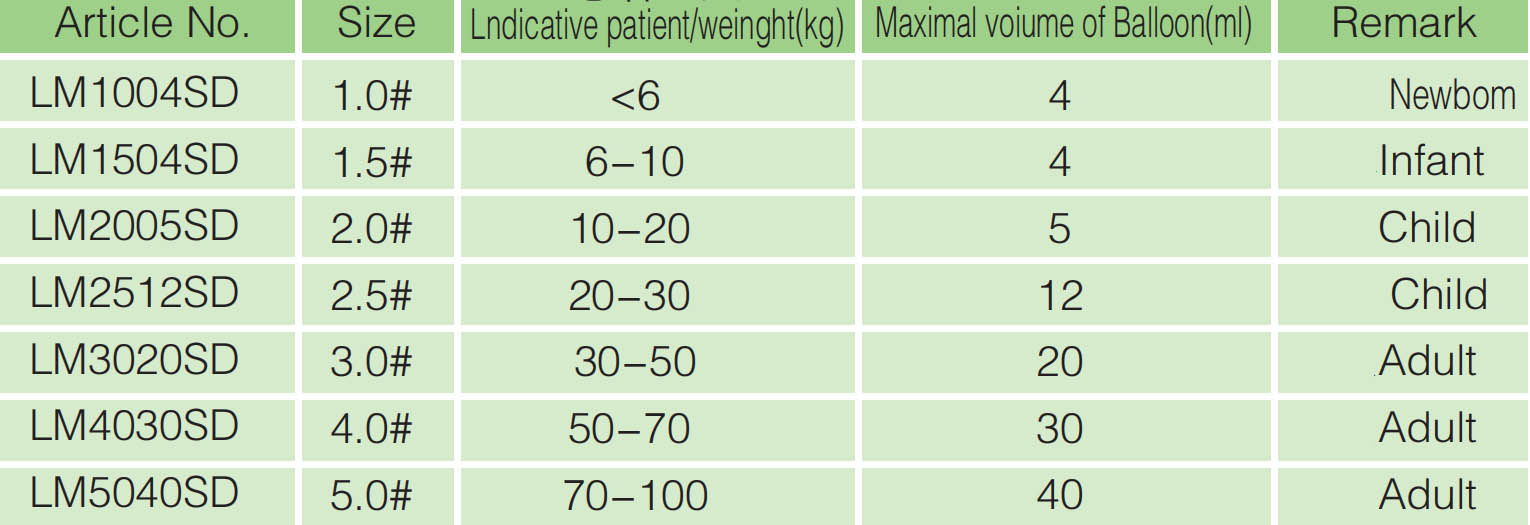
Model |
Ordinary type, Reinforced type, |
|||||||
|
Specifications(#) |
1 |
1.5 |
2 |
2.5 |
3 |
4 |
5 |
6 |
|
Maximum inflation(Ml) |
4 |
6 |
8 |
12 |
20 |
30 |
40 |
50 |
|
Applicable patient / body weight(kg) |
Neonatus<6 |
Baby6~10 |
Children10~20 |
Children20~30 |
Adult30~50 |
Adult50~70 |
Adult 70~100 |
Adult >100 |
1. The LMA, should check with the specifications of product labeling.
2. To exhaust the gas in the airway of the laryngeal mask airway so that the hood is completely flat.
3. Apply a small amount of normal saline or water soluble gel for lubrication in the back of the throat cover.
4. The patient's head was slightly back, with his left thumb into the patient's mouth and traction of the patient's jaw, in order to widen the gap between the mouth.
5. Using the right hand to hold the pen holding the laryngeal mask, to make available, the index finger and middle finger finger against the cover connection body and the ventilation tube laryngeal mask, cover the mouth towards the direction along the midline of lower jaw, tongue sticking down pharyngeal LMA, until no longer advance so far. Also can use reverse the method of inserting laryngeal mask, just cover the mouth toward the palate, will be placed in the mouth to the throat at the bottom of the laryngeal mask, and 180O after rotation, and then continue to push down the laryngeal mask, until can't push so far. When using the enhanced or ProSeal laryngeal mask with guide rod, the guide rod can be inserted into the air cavity to reach the designated position, and the insertion of the laryngeal mask can be drawn out after the insertion of the laryngeal mask.
6. In the move before the other hand gently with finger pressing to prevent laryngeal mask airway catheter displacement.
7. According to the nominal charge to cover bag filled with gas (air amount can not exceed the maximum filling mark), connect the breathing circuit and assess whether good ventilation, such as ventilation or obstruction, should according to the steps of re insertion of laryngeal mask.
8. To confirm the position of the laryngeal mask is correct, cover the tooth pad, fixed position, maintain ventilation.
9. The throat cover is pulled out: the air behind the air valve of the syringe with the syringe without needle is pulled out of the throat cover.
1. Patients who were more likely to have a full stomach or stomach content, or who had a habit of vomiting and other patients who were prone to reflux.
2. Abnormal enlargement of the patient with bleeding in the respiratory tract.
3. The potential of respiratory tract obstruction patients, such as sore throat, abscess, haematoma etc.,
4. The patient is not suitable for the use of this product.
1. Before use should be based on age, body weight of different choice of the correct model specifications and detect whether the bag leak.
2. Please check before use, such as found in single (packaging) products have the following conditions, the prohibition of the use of:
a) Effective period of sterilization;
b) The product is damaged or has a foreign body.
3. Use should observe the patient thoracic activity and auscultation of bilateral breath sound to determine the ventilation effect and end expiratory carbon dioxide monitoring. Such as the discovery of thoracic or poor or non fluctuating amplitude fluctuations hear the leak sound, should immediately pull the laryngeal mask, after full oxygen again after implantation.
4. Positive pressure ventilation, airway pressure should not exceed 25cmH2O, or prone to leakage or gas into the stomach.
5. Patients with laryngeal mask should be fasting before use, in order to avoid the possibility of anti - flow induced aspiration of gastric contents during positive pressure ventilation.
6. This product is ethylene oxide sterilization, sterilization is valid for three years.
7. When the balloon is inflated, the amount of charge should not exceed the maximum rated capacity.
8. This product for clinical use, operation and use by medical personnel, after the destruction.
[Storage]
Products should be stored in relative humidity of not more than 80%, the temperature is not more than 40 degrees Celsius, no corrosive gases and good ventilation clean room.
[Date of manufacture] See inner packing label
[expiry date] See inner packing label
[Specification publication date or revision date]
Specification publication date : September 30, 2016
[Registered person]
Manufacturer: HAIYAN KANGYUAN MEDICAL INSTRUMENT CO., LTD

 中文
中文