Recently, Haiyan Kangyuan Medical Instrument Co., Ltd. Produced breathing circuits for single use, in the provincial supervision inspection organized by the Zhejiang Provincial Market Supervision Administration, with excellent product quality, successfully passed all the inspection items, inspection report number: Z20240287.

The inspection was carried out by the Hangzhou Medical Device Quality Supervision and Inspection Center of the State Food and Drug Administration. The comprehensive and detailed inspection was carried out in strict accordance with the sampling plan and product technical requirements of the respiratory pipeline products for anesthesia machines and ventilators in document [2024]1 of Zhejiang Provincial Medical Device Quality Sampling Inspection Plan and Annex 2 of the Plan for the Supervision Sampling and Inspection of Medical Devices of Zhejiang Province in 2024. The inspection project covers the appearance, length, airflow resistance, leakage and other key indicators of the breathing circuits, ensuring that every detail of the product meets the national standard.
Kangyuan Medical always regards product quality as the lifeline of the enterprise, and insists on implementing strict quality control measures in every link from raw material procurement, production and processing to finished products. Kangyuan Medical has a high-quality R & D and production team, as well as advanced production equipment and testing technology, which provides a solid guarantee for the production of high-quality medical equipment products (such as silicone foley catheter, silicone foley catheter with temperature probe, laryngeal mask airway, tracheostomy tube, anesthesia mask, nebulizer mask, oxygen mask, breathing filter, suction-evacuation access sheath for single use, etc.).
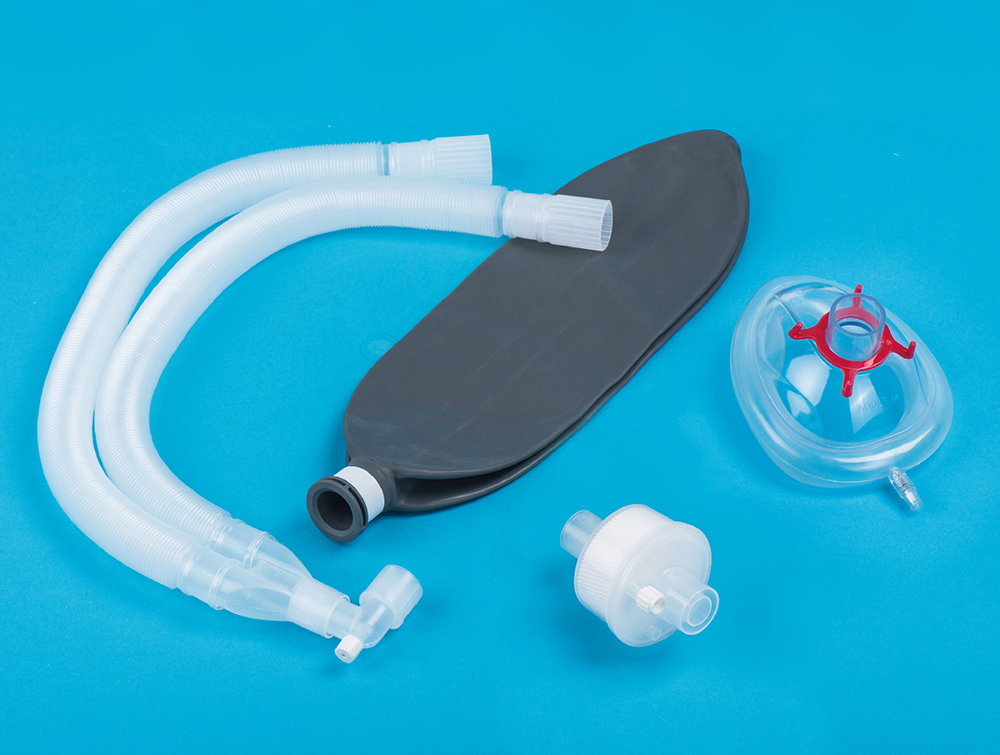
The passing of this random inspection not only proves the competitiveness of Kangyuan Medical in the medical device market, but also provides a safer and more reliable choice of medical products for the majority of medical institutions and patients. In the future, Kangyuan Medical will continue to be committed to medical consumables technology innovation and product upgrades, and strive to promote the continuous progress and development of the medical device industry.
Post time: Jul-22-2024

 中文
中文